Current project updates, seed concept pitches for 2022, and the future direction of the Center for Bioplastics and Biocomposites were all in focus as the Center, an Industry & University Cooperative Research Center funded by the National Science Foundation, held its virtual spring Industry Advisory Board meeting in May. The UGA New Materials Institute serves as one of four university research sites for CB2, along with Washington State University, Iowa State University, and North Dakota State University.
CB2 leadership told members they currently await approval on a grant proposal, submitted late fall to the NSF, to elevate all four sites and the Center to a Phase II research cooperative. A Phase II IUCRC requires a larger industry consortium, which will yield a wider range of projects for annual consideration, thereby increasing opportunities for researchers and students, along with the output of shared intellectual property benefitting IAB member companies. CB2 expects an answer from the NSF later this spring.
Experiential learning
For students, CB2 opens doors to research experiences, professional mentorships, and potentially future jobs, working in collaboration with scientists and product developers from some of the biggest names in industry: Amazon, Ford, John Deere, ADM, Kimberly-Clark, 3M, BASF, Boehringer Ingelheim, Sherwin-Williams, AkzoNobel,Danimer Scientific, NatureWorks, RWDC Industries, and Avery Dennison, among others. When funded as a Phase II IUCRC, CB2 will expand its scope to include recycling/circularity and end-of-life scenarios for materials—both research strengths at the UGA New Materials Institute. Industry’s need to resolve problems in recycling streams and to increase circularity in their material supply chains was reflected in some of the projects pitched by IAB members.
Overall, 14 seed concepts were pitched and will be further defined through the coming weeks by IAB members and researchers. The IAB will vote this fall on what projects to fund for 2022. The seed concepts range from exploring new materials for coatings, packaging and textiles; to characterizing the properties of new polymer sources to gain a better understanding of potential applications for these materials; to improving the quality of materials that enter recycling streams by reducing contamination, and through better screening of the materials in these streams.
UGA project updates
In updates on existing projects, UGA researchers again got good feedback from IAB members on the progress made thus far and the plans toward completion of their projects, which are:
“Unlocking the Potential of Bidegradable Xylan-based Polymer Materials,” led by Breanna Urbanowicz, an assistant professor in the Department of Biochemistry and Molecular Biology, in the Franklin College of Arts and Sciences, and a member of the Complex Carbohydrate Research Center, who researches the structure and functionality of plant carbohydrate active enzymes. The first two years of this project were dedicated to exploring the potential of xylans, and later mannans, and the best routes to functionalize these polymers. Now in year 3, the team is exploring catalysts, investigating functionalization for upstream and downstream uses, and continuing to build its saccharide library. Xylans and mannans are two of the most abundant polymers in nature and in agricultural waste streams.
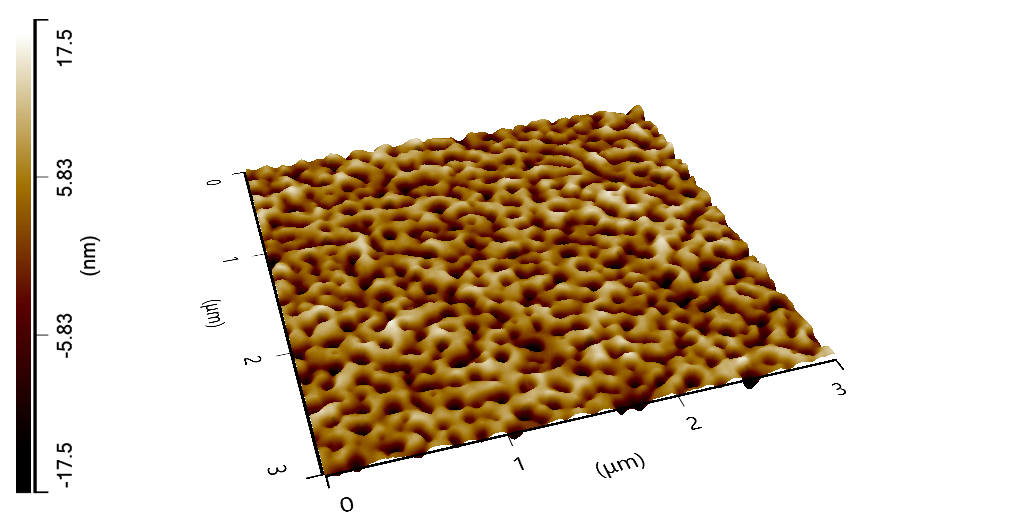
“Investigating the Enzymatic Degradability of Glycolic-Urethane Linkages,” led by Evan White, an assistant research scientist and director of the New Material Institute’s Bioseniatic℠ Laboratory. This project, in its second year, aims to develop a rapid screening assay to evaluate materials for their ability to be deconstructed by enzymes at the end of their useful life, which may analyze hundreds of candidate materials compared to selective and time intensive in vitro testing such as respirometry or disintegration. White’s team has honed a test to provide reliable data within 8 hours. The team will validate the rapid screening assay with concomitant analyses such as respirometry, and aims to publish the analytical method to help expedite the discovery of new compostable materials.
“Life Cycle Assessment Tool for Sustainable Bio-based Coating Material Design,” led by Ke Li, an associate professor based in the College of Engineering. This is the first LCA tool to be developed for CB2’s IAB. To date, results from LCAs have been contradictory for the environmental benefit of bio-based plastics, making it difficult for manufacturers to thoroughly evaluate a proposed material’s overall sustainability and compare it to the overall life cycles of other materials. In the early months of his project, Li identified factors that have historically contributed to uncertainty in LCAs of bio-based materials, and identified various hot spots for different bio-based products. In the months ahead, he will develop an approach to quantify the uncertainty and collect the data accordingly to reduce the uncertainty of LCA and develop a streamline tool for industries of bio-based plastics.
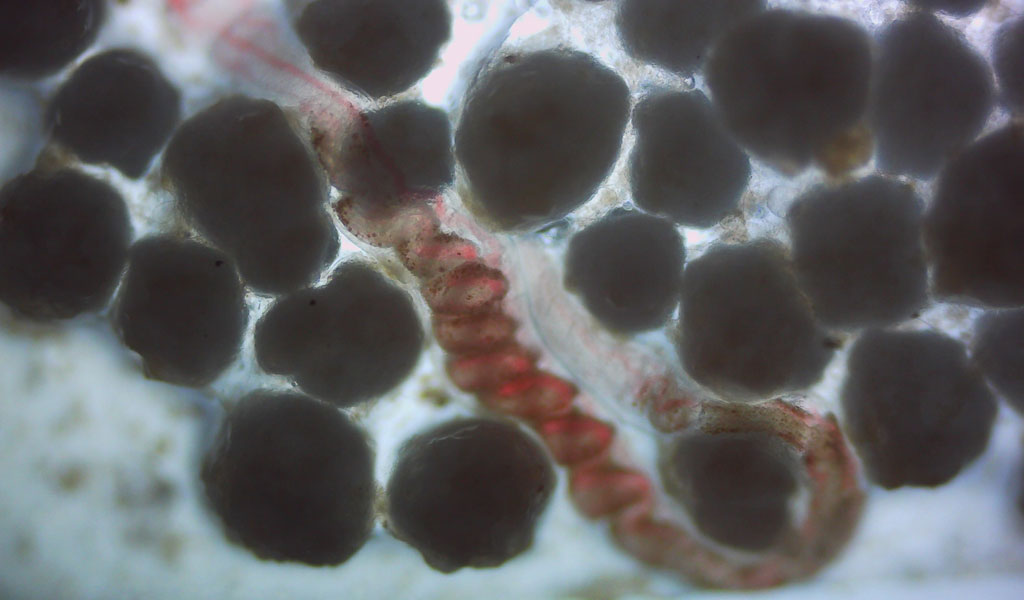
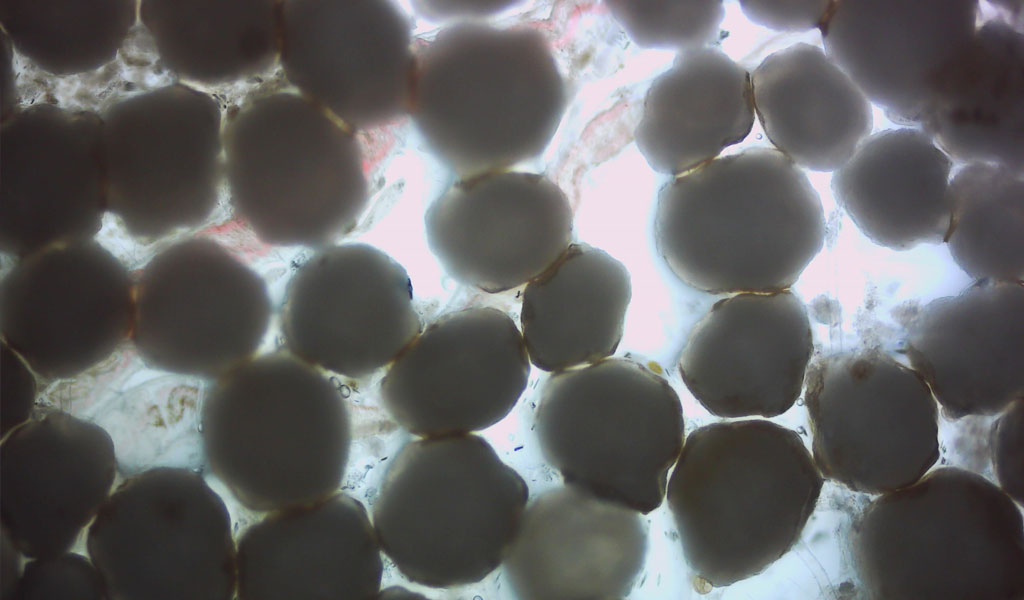
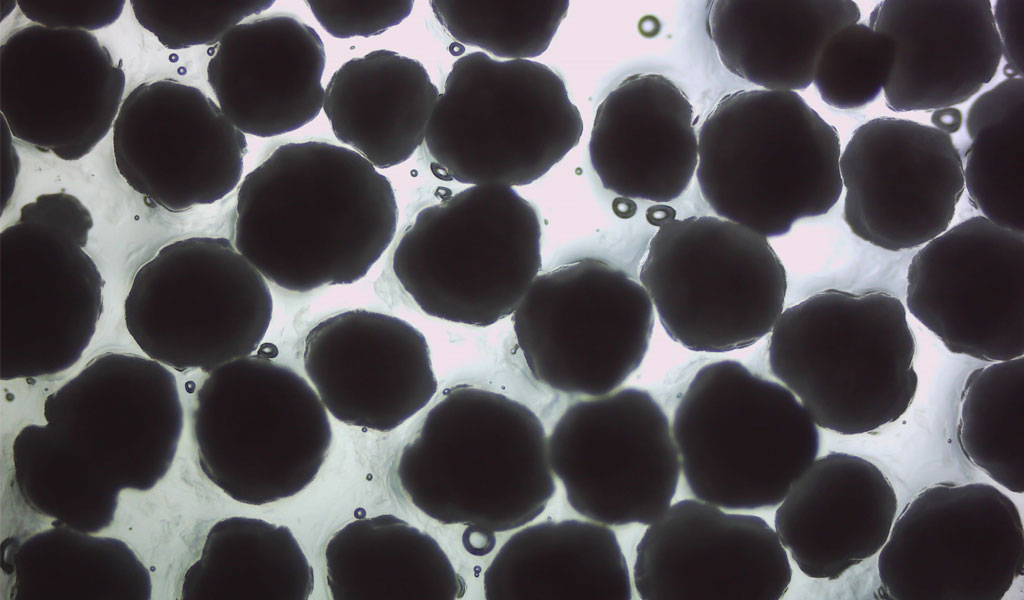
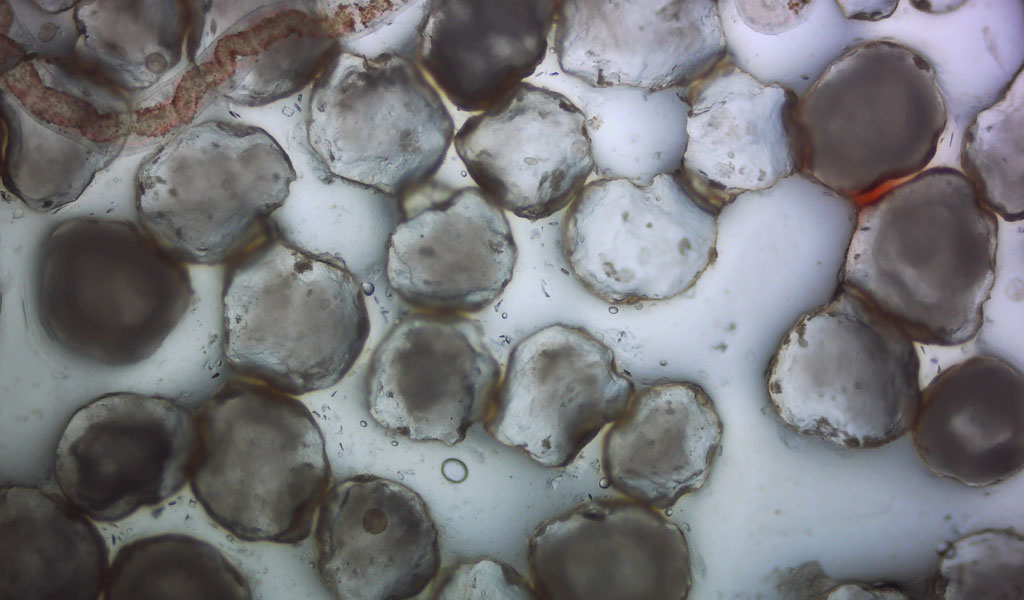
“Investigation of the Marine Degradability of Polymers of Interest to IAB Members,” led by Dr. Branson W. Ritchie, a Distinguished Research Professor who leads the UGA Infectious Diseases Laboratory and heads Technology Development and Implementation for the New Materials Institute. This project will start in mid-year 2021 and involves both respirometry and field testing.
The UGA New Materials Institute’s participation as a research site for CB2 is supported by NSF Award #1841319.